The ever-increasing demand for medical device assembly and medtech requires market-proven, world-class manufacturing technologies from a trusted assembler. Digital technology for greater efficiency, faster to market.


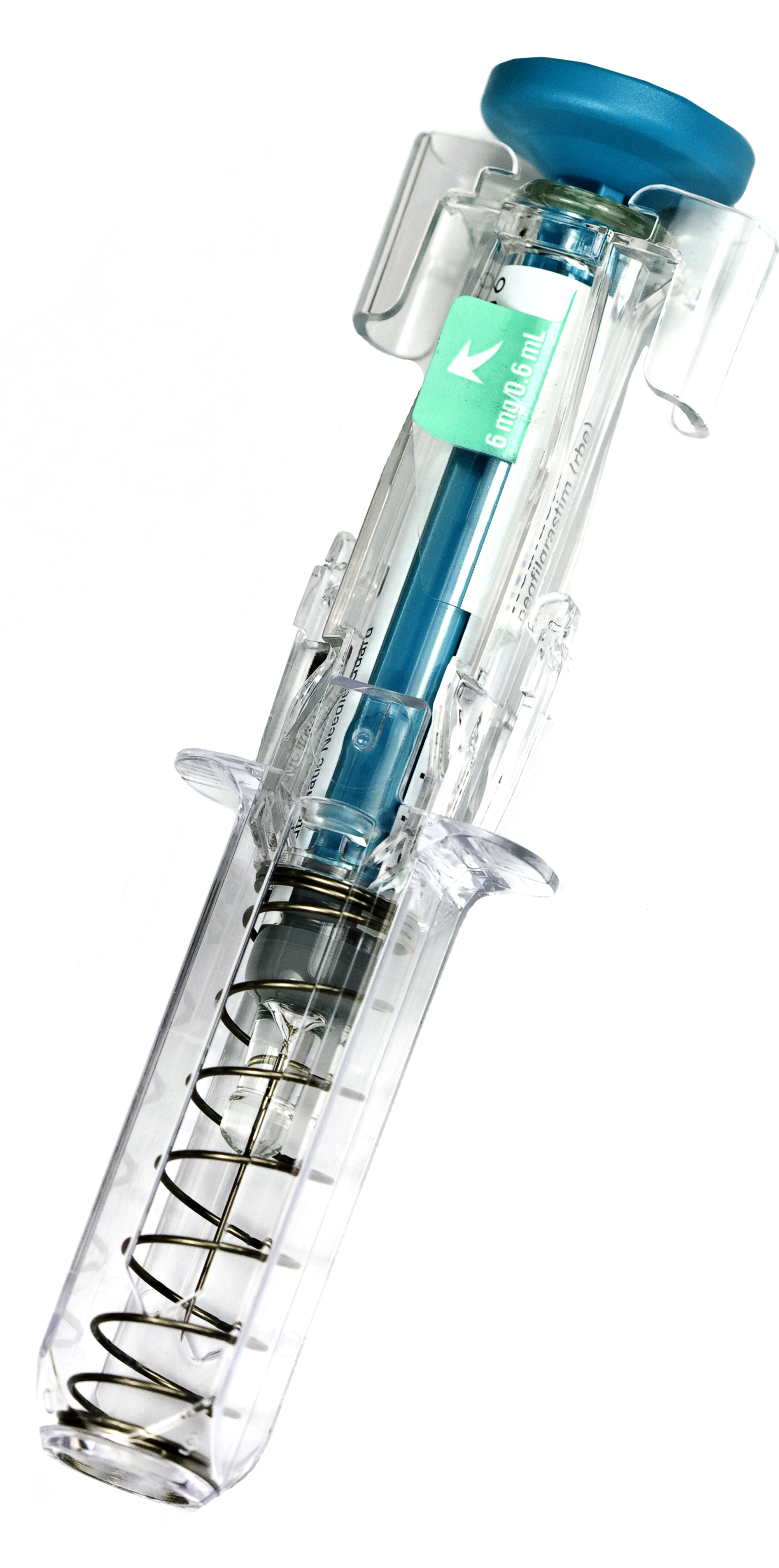


YpsoMate® is a registered trademark of The Ypsomed Group.
SYMPHONI is an innovative and proven digital assembly platform with a configurable, modular design resulting in a smaller factory footprint. Not only is it space-saving, but it is also flexible, which minimizes tooling requirements and eliminates non-value-added motion time, resulting in cost savings. Incorporating SYMPHONI as part of your assembly process is low risk as it is designed for long-term deployment to support ongoing capacity ramp-up.
SYMPHONI goes fast where it can and slow where it must. Critical process times are never compromised. SYMPHONI Technology’s enhanced servo-based motions and e-Cams mean a single system can run multiple product lines 24/7 with 90% less retooling. The compact design minimizes cleanroom footprint, and its standardization simplifies validation.
Driving Success through Innovation
A client approached ATS to see if there was a better way to assemble autoinjectors faster and more efficiently. They wanted speed, flexibility, and performance all wrapped into one. They wanted their core processes to be respected while running two different autoinjectors on the same machine.
Download our Autoinjector Series Brochure to learn more
The outcomes were clear, powerful, and—from the client’s perspective—groundbreaking. Symphoni dramatically reduced footprint by more than 50%, reduced tooling by over 90% and increased quality without sacrificing performance. [Continue Reading]
Driving Success through Innovation
For medical device manufacturers, scaling production is not just about building a functional production line but also about making sure that millions of devices are produced flawlessly. Yet, many conventional assembly processes remain a “black box,” with quality assurance limited to pre- and post-assembly inspections. These legacy methods create significant challenges: they consume valuable floor space and require numerous specialized tools. To meet stringent standards such as FDA 21 CFR Part 11 and EU MDR, manufacturers must demonstrate consistent product quality providing fully traceable and audit-ready process data. Optimized layouts and minimal downtime are also key factors.
Combining advanced sensor technology from Kistler with the SYMPHONI™ patented digital motion system from ATS Life Sciences Systems (LSS), SYMPHONI™ delivers real-time in-line quality assurance for medical devices, up to 320 parts per minute with 90 percent less tooling and full traceability. The platform sets a new benchmark for scalable, data-driven automation in medical device manufacturing, supporting regulatory-compliant production, audit-ready data, and flexible handling of multiple product types on a single line. These improvements can reduce overall cycle time from 3750 ms to 750 ms, delivering rapid, precise, and traceable in-line process monitoring, with up to 50% less footprint. [Continue Reading]
Tell me more
Find out how the SYMPHONI assembly platform can work for your application